Triple winning
combination
BIO
PCR amplifies all pathogens
Screening via hybridization

We find what we are not looking for
Molecular biology allows the identification of pathogens by their genotype (DNA) in place of the phenotypic identification of microbiology.
Dendris DNA microarray technology is based on a syndromic approach. Microbiology or PCR generally targets a few microorganisms per analysis. The syndromic approach aims to simultaneously identify all pathogens in a single analysis, based on symptoms allowing improvement in diagnosis and patient care.
A multiplex consensus PCR will amplify all pathogens present in the sample to be analyzed.
Hybridization will screen and identify pathogens present thanks to a combination of specific probes to each target.
The key points of DNA microarray technology are the sensitivity and specificity of the interaction between the probes and the target, which result from the design of the probes and the chemistry that binds the probes to the support. Our technology is based on the functionalization of the support with phosphorus dendrimers, which significantly increases the sensitivity of our chip.
NANO
Surface chemistry by dendrimers
Ratio improvement
signal/noise

INFO
AI profile analysis
Centralized database
The analysis of one benefits the results of the other for better patient care by clinicians
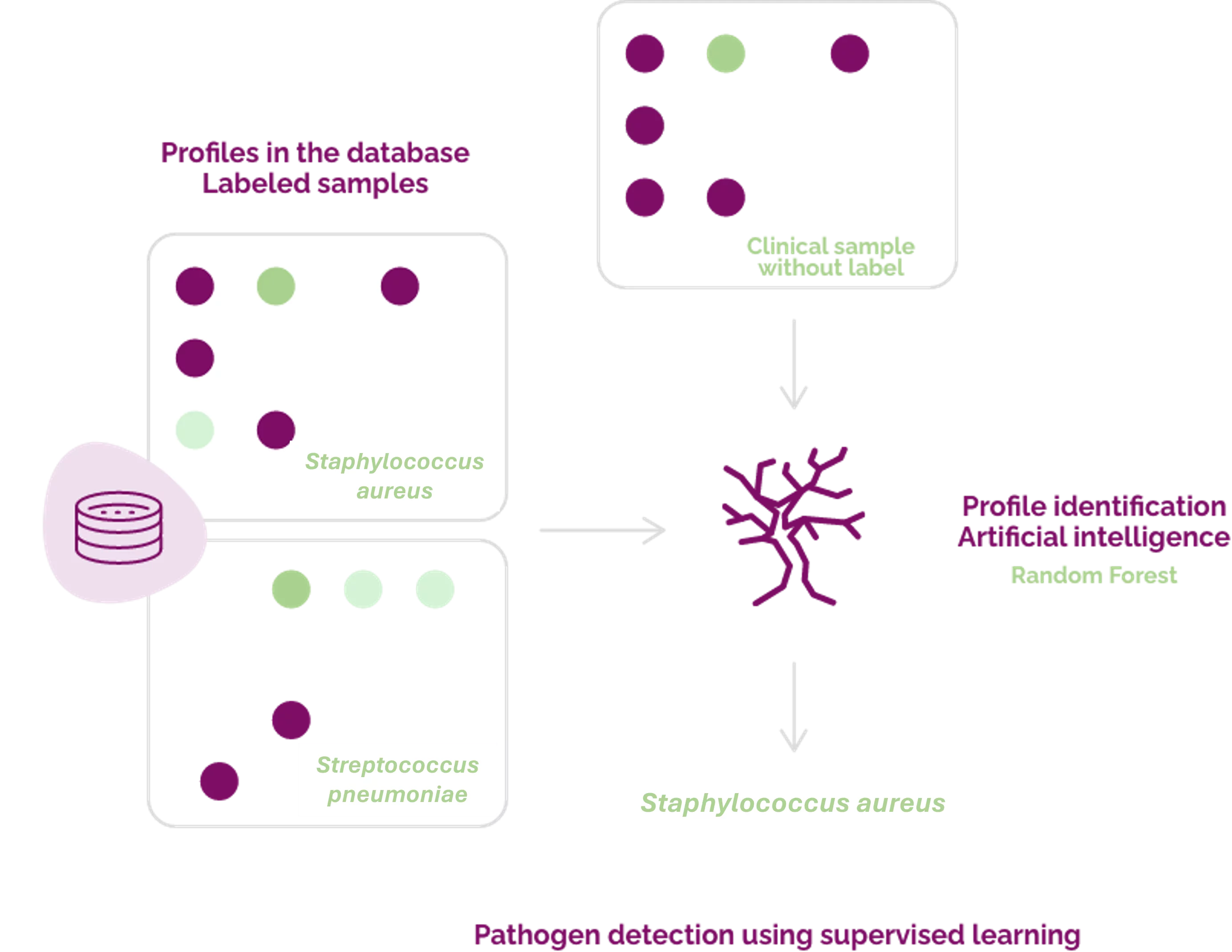
Dendris uses a machine learning algorithm to deliver the results of analyzed clinical samples. From a learning base of labeled samples (supervised) our algorithm will build its model. From this model it will be able to provide predictions on new samples in an automatic, reliable and fast way.
The algorithm selected by Dendris is based on the combination of decision trees. This algorithm is called Random Forest. This program is widely used in artificial intelligence (AI) to solve prediction problems, especially in the field of health (1832 citations on PubMed in 2022).
Process Dendris
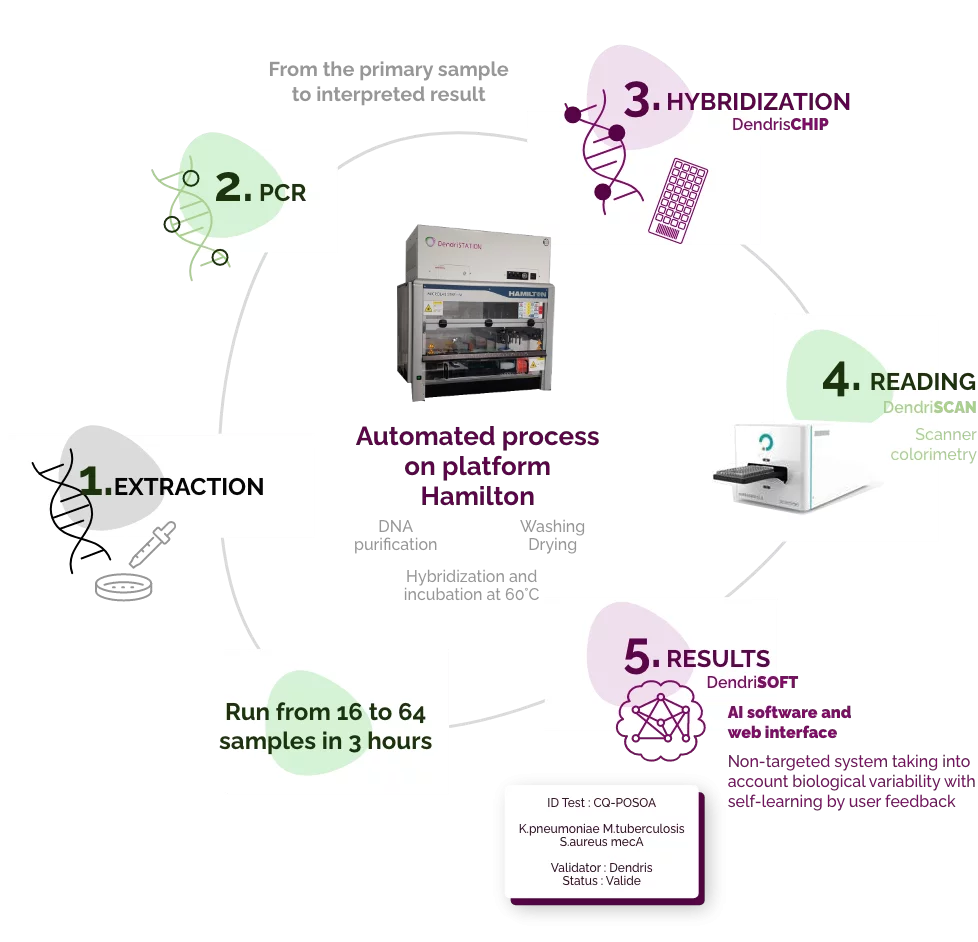
From 16 to 64
analyzes per run
The automation of our solution is one of the priority points to fully meet the productivity needs of our testing laboratories. This step will reduce analysis costs, increase the robustness and reliability of our solution while retaining the advantages already noted by laboratories: sensitivity and quick results.
Powerful,
secure and productive tool
At the same time, the development of various applications such as respiratory infections (viruses and bacteria), invasive mycosis (fungi and yeast), vaginosis, etc. will allow us to offer a complete range of products to all our users.
Les Dendrimers
Our team leaded by Jean-Pierre MAJORAL, world class expert in “Design and properties of dendrimers from biology and medicinal chemistry to material sciences, and catalysis” offers high level services in design and manufacturing of dendrimers fitted to your needs.
Contact us to learn more on our chemistry product and services.
Dendrimers are “perfectly defined arborescent macromolecules whose structure starts from a central core and goes via branching units to peripheral functions”.
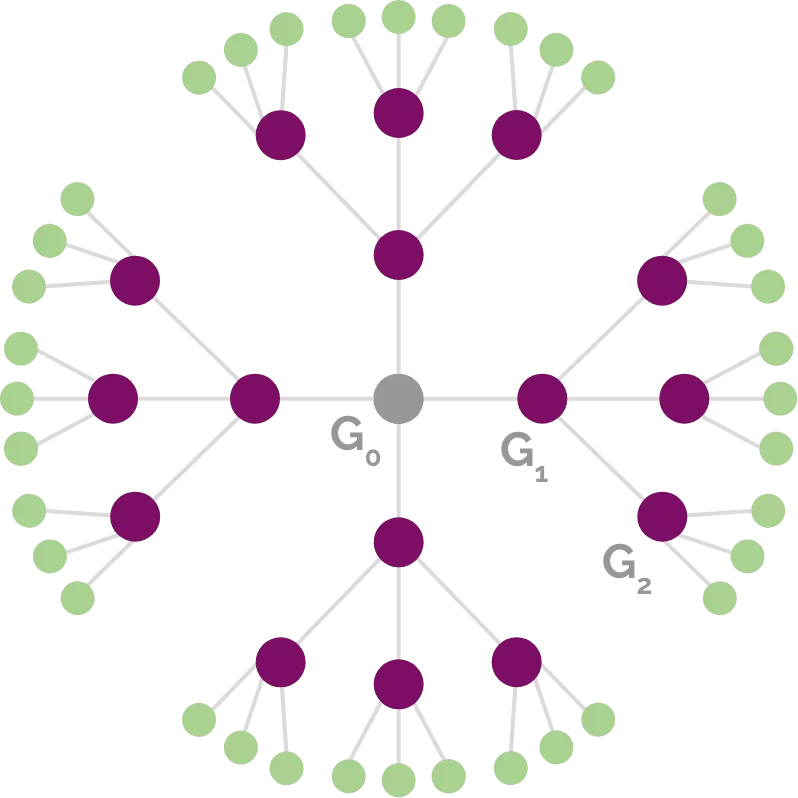
Central core
Branching unit
Peripheral function
Generation

A lot of molecules can be used to act as branching units, making it possible to build as many different dendrimers as there are applications
Phosphorus dendrimers were first used in biochips in 1999. Since then, many systems have been developed to improve results quality and biochips sensitivity.
In Dendris, phosphorus dendrimers are used as spacers between the surface and the oligonucleotide probes. However, the presence of phosphorus atoms at each branching point offers the possibility to perform a large number of reactions and thus to prepare a large variety of macromolecules whose structure is perfectly controlled. These compounds are used in many fields: catalysis, electronics, nanomaterials, biology (anti-HIV activity, Alzheimer’s, oncology, imaging, etc.).
Machine learning

Dendris is the holder of the data towards the customer
No patient data
Health data storage by an approved provider at XEFI
Dendris uses a machine learning algorithm to deliver results from analyzed clinical samples. From a learning base made up of labeled (supervised) samples, the algorithm will build its model. From this model it will be able to provide predictions on new samples automatically, reliably and quickly.
The algorithm chosen by Dendris is based on the combination of decision trees. This algorithm called Random Forest was proposed in 2001 by Leo Breiman, member of the Academy of Sciences in the USA. This program is widely used in Artificial Intelligence (AI) to solve prediction problems, particularly in the field of health (1832 citations on PubMed in 2022).
Since the creation of Dendris in 2009, we have adapted and parameterized the Random Forest in order to better exploit the data generated by the DendrisCHIP biochip. This constantly improved AI allows us to meet our customers’ expectations with optimal sensitivity and specificity of pathogen detection, making it a powerful tool for treating patients. Samples transferred to the application enrich the learning base allowing the model to become more and more efficient and to enlarge the panels with new targets. Our application and data are hosted on servers (dual site) certified as health data hosts (HDS) by our expert partner XEFI. The HDS certification guarantees security through data encryption and a strict confidentiality policy.

